Home » Aside » Triple Point of Water
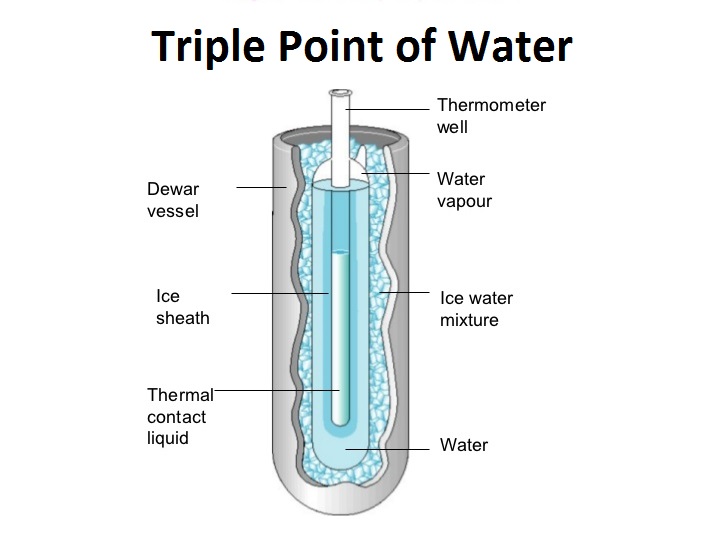
The triple point of a substance is the temperature and pressure at which three phases i.e the liquid, gas, and solid of that substance may coexist in thermodynamic equilibrium.
The single combination of pressure and temperature at which liquid water, solid ice, and water vapour can coexist in a stable equilibrium occurs at exactly 273.16 K (0.01 °C; 32.02 °F) and a partial vapour pressure of 611.657 pascals.
At that point, it is possible to change all of the substance to ice, water, or vapour by making arbitrarily small changes in pressure and temperature.
Daksha is an integral part of the editorial team at Zigya. Armed with a B.Tech degree, she oversees content quality assurance for Biology. Her subtle wit, observation skills and agile demeanour bring the buzz in the editorial team and ensures meeting stiff deadlines. An astute blogger, when not working Daksha prefers to spend her time with her canine companion, Spiky.
Follow her work at www.zigya.com
More Posts
Daksha is an integral part of the editorial team at Zigya. Armed with a B.Tech degree, she oversees content quality assurance for Biology. Her subtle wit, observation skills and agile demeanour bring the buzz in the editorial team and ensures meeting stiff deadlines. An astute blogger, when not working Daksha prefers to spend her time with her canine companion, Spiky.
Follow her work at www.zigya.com
Published in Interesting Facts and Physics
Nice work. Keep it up