CBSE
Class 10 Class 12
Body-Centred Unit Cells: A unit cell contains one constituent particle (atom, molecule or ion) at its body-centre and eight particles are at its corners.
Number of atoms present in the unit cell =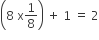
Thus, Number of lattice points: 9, coordinate number:er : 8 and number of atom per unit cell (Z): 2
Body-Centred Unit Cells: A unit cell contains one constituent particle (atom, molecule or ion) at its body-centre and eight particles are at its corners.
Number of atoms present in the unit cell =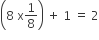
Thus, Number of lattice points: 9, coordinate number:er : 8 and number of atom per unit cell (Z): 2
End-Centred Unit Cells:Unit cell contains one constituent particle present at the centre of any two opposite faces along with eight particles at its corners.
Number of atoms present in this unit cell = 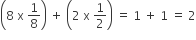
Thus, Number of lattice points:10
Face-Centred Unit Cells: A unit cell contains one constituent particle present at the centre of each face, along with eight particles at its corners.
Number of atoms present in this unit cell = 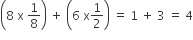
Thus, number of lattice points: 14, coordination number: 12 and number of atom per unit cell (Z): 4
Primitive Unit Cells: When constituent particles are present only on the corner position of a unit cell.
No of atoms present in this unit cell = 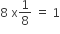 (Each Corner atom contributes1/8th portion to the unit cell Thus,
(Each Corner atom contributes1/8th portion to the unit cell Thus,
Number of lattice points: 8, Coordination Number: 6 and number of atom per unit cell (Z): 1
Centred unit cells: Constituent particles present at a position other than corners in addition to those at corners, it is called a centred unit cell.
Centred unit cells are of three types: