CBSE
Class 10 Class 12
1) Mass percentage (w/w): The mass percentage of a component of a solution is defined as:
 2) Volume percentage (v/v): The volume percentage is defined as:
2) Volume percentage (v/v): The volume percentage is defined as:

3) Mass by volume percentage (w/v):

Common units for w/v% concentration are g/100mL (%)
4) Parts per million: When a solute is present in trace quantities, it is convenient to express concentration in parts per million (ppm) and is defined as:

5) Mole fraction of a component :

The used symbol for mole fraction is x and subscript used on the right-hand side of x denotes the component.
For binary solution: The number of moles of A and B are nA and nB respectively so,
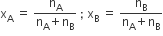
In binary solution xA + xB =1
6) Molarity: Molarity (M) is defined as a number of moles of solute dissolved in one litre (or one cubic decimetre) of the solution.

Unit of Molarity are mol L-1, mol dm-3
7) Molality: Molality (m) is defined as the number of moles of the solute per kilogram (kg) of the solvent and is expressed as:

Unit of molality are mol kg-1
8) Normality (N) is defined as the number of mole equivalents per liter of solution:

Molarity (M), Molality(m) relates the amount of solute to the total volume of solution; however, normality is specifically used for acids and bases.