CBSE
Class 10 Class 12
The pressure of the vapour above the liquid at equilibrium in a closed container refers to as the vapour pressure of the liquid. Vapour pressure have a characteristic value at a particular temperature and increases with temperature.
The vapour pressure of a liquid at a given temperature can be defined as the pressure of the vapour in equilibrium with the liquid at that temperature in an enclosed space.
The magnitude of the equilibrium vapour pressure depends on:
Azeotropes are binary mixtures possessing the same synthesis in fluid, vapour phase and boiling stage at a constant temperature.
The components of azeotropic mixtures are separated by fractional distillation.
Azeotropes can be categorized into two types-
Minimum boiling azeotrope: The solution which shows a large positive deviation from Raoult's law. Example- ethanol-water mixture.
Maximum boiling azeotrope: The solution which shows large negative deviation from Raoult's law. Example- Nitric acid - water mixture.
It states that for a solution of a volatile liquid, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution.
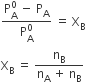
The total pressure is equal to the sum of partial pressure.
Ptotal= PA + PB
2) For a solution containing non-volatile solute, the vapour pressure of the solution is directly proportional to the mole fraction of the solvent.
Solids dissolved in liquid, for example, sodium chloride, glucose, urea and cane sugar in water and iodine and sulphur dissolved in carbon disulphide.
Addition of solid solute in the solvent decreases the vapour pressure of the solutions. This vapour pressure is lower than the vapour pressure of the pure solvent at a given temperature.
In the solution, the surface has both solute and solvent molecules; thereby the fraction of the surface covered by the solvent molecules gets reduced.
The decrease in the vapour pressure of solvent depends on the quantity of non-volatile solute present in the solution, not on its nature.