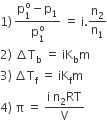CBSE
Class 10 Class 12
Molar mass that is either lower or higher than the expected or normal value is called as abnormal molar mass.
Van’t Hoff introduced a factor i, known as the van’t Hoff factor, to account for the extent of dissociation or association. This factor i is defined as:
Ratio of normal molecular mass to the observed molecular mass of the solute.

Van’t Hoff factor modifies the equations for colligative properties as follows: