Short Answer Type
Short Answer TypeArrange the following in order of increasing basic strength.
Aniline, ethylamine, ethane, phenol.
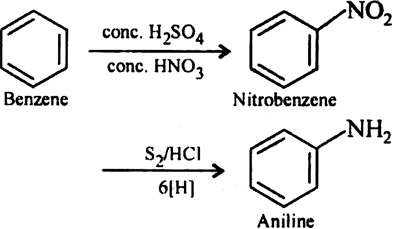
Benzene reacts with the nitric acid in presence sulphuric acid to form nitrobenzene and further reaction with S2/HCl to give aniline.