 Short Answer Type
Short Answer Type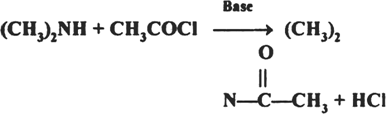
The acid generated in the reaction can form a salt of the amine which thus will lose nucleophilic character and the reaction will not proceed to completion.
(CH3)2NH + HCl → (CH3)2NH2⊕ClΘ
So, a base is needed to facilitate the reaction.
How is the basic strength of aromatic amines affected by the presence of an electron releasing group on the benzene ring ?
Which compound is formed when benzene diazonium chloride reacts with phenol in basic medium?
