 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeIdentify the substances A and B in each of the following sequences of reactions:
CH3CONH2 is a weaker base than CH3CH2NH2
Explain the following reactions by taking one suitable example in each case:
Hoffmann's bromamide reaction.
Explain the following reactions by taking one suitable example in each case:
Gattermann’s reaction.
How is ethylamine prepared from acetonitrile? Compare its basic character with that of ammonia.
 Long Answer Type
Long Answer TypeDescribe the method for the identification of primary, secondary and tertiary amines. Also write chemical equations of the reactions involved.
The three types of amines can be distinguished by Hinsberg method. The sample is treated with benzene sulphonyl chloride, C6H5SO2Cl (Hinsberg’s reagent) followed by treatment with aqueous KOH (5%) solution. Based upon the observations, the following conclusions may be drawn:
(i) If a clear solution is obtained , then it is a primary amine.
(ii) If the solution is turbid or ppt appears and remains unaffected by the addition of an acid, the given amine is a secondary amine.
(iii) If the sample remains insoluble in alkali and dissolves in an acid, then it is a tertiary amine.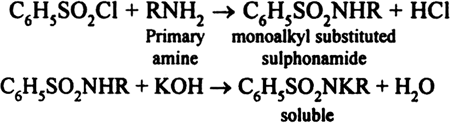
The secondary amine forms dialkyl sulphonamide which is insoluble in alkali![]()
Tertiary amine do not react with Hinsberg’s reagent. They do not dissolve in alkali, but dissolve in acid.![]()
 Short Answer Type
Short Answer Type