 Short Answer Type
Short Answer TypeGive plausible explanation for each of the following:
Why are amines less acidic than alcohols of comparable molecular masses?
Oxygen in alcohol is more electro negative than nitrogen in amines. RC —H bond in alcohol is more polar with δ+ charge on
ROδ-Hδ+ as compared to RN—H bond in Alcohols can loose proton to some extent but are proton acceptors.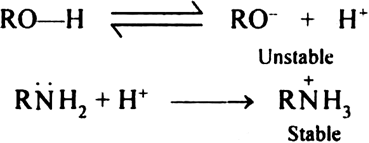
Give plausible explanation for each of the following:
Why are primary amines higher boiling than tertiary amines?
Give plausible explanation for each of the following:
Why are aliphatic amines stronger bases than aromatic amines?
