 Short Answer Type
Short Answer TypeExplain how does the presence or absence of hydrogen on N of amines affect the modes of their reactions with nitrous acid?
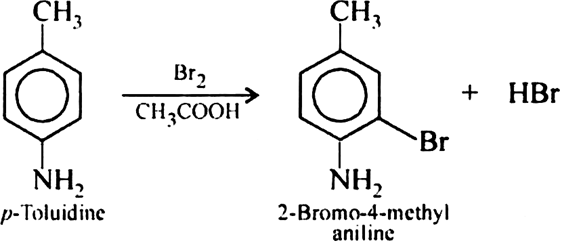
Arrange the isomeric compounds:
(i) Ethyldimethyl amine
(ii) n-butyl amine
(iii) diethyl amine
in order of decreasing boiling point and give reason.
Explain the following general order of basicity in aqueous solution:
R2NH > RNH2 > R3N > NH3
Explain the following general order of basicity in aqueous solution:
R3N ,R2NH , RNH2
What will be the basic strength order of these amines in gas phase?
How can you find out whether a given amine is a primary amine? Write the chemical reaction involved in the test you perform.
 Long Answer Type
Long Answer TypeHow can you separate a mixture of primary, secondary and tertiary amines? Write chemical reactions involved in the process.
