 Multiple Choice Questions
Multiple Choice QuestionsReaction of an aliphatic primary amine with nitrous acid in cold leads to the formation of
Amongst the following, the most basic compound is
Aniline is heated with conc. H2SO4 at 460-475 K, the product formed is
 Short Answer Type
Short Answer Type
 Multiple Choice Questions
Multiple Choice QuestionsBenzene diazonium chloride when reacts with hypophosphorous acid produces
Which of the following has the higher value of Kb (base dissociation constant)?
Which of the following product is obtained when m-dinitro benzene is reduced within tin and hydrochloric acid?
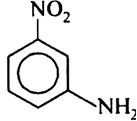
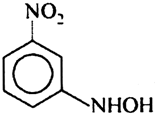
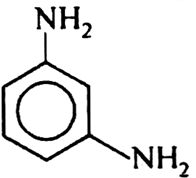
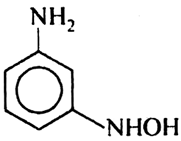
 Short Answer Type
Short Answer Type