 Short Answer Type
Short Answer TypeComplete the following chemical reaction equations:
(i) C6H5N2Cl + H3PO2+ H2O --->
(ii) C6H5NH2+ Br2(aq.)--->
Describe the following giving the relevant chemical equation in each case:
(i) Carbylamines reaction
(ii) Hofmann’s bromamide reaction.
Write the chemical equations involved in the following reactions:
i) Hoffmann-bromamide degradation reaction
ii) Carbylamine reaction
Give reasons for the following:
i) Aniline does not undergo Friedal-Crafts reaction.
ii) (CH3)3N is more basic than (CH3)3N in an aqueous solution.
iii) Primary amines have higher boiling point than tertiary amines.
Give a chemical test to distinguish between ethylamine and aniline.
Ethylamine and aniline can be distinguished from each other by the azo-dye test.
A dye is obtained when aniline (an aromatic amine) reacts with HNO2 (NaNO2 + dil.HCl) at 0-5°C, followed by a reaction with the alkaline solution of 2-naphthol.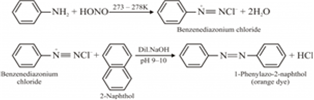
Ethylamine (an aliphatic amine) gives a brisk effervescence due (to the evolution of N2 gas) under similar conditions.
