 Short Answer Type
Short Answer TypeState reasons for the following:
(i) pKb value for aniline is more than that for methylamine.
(ii) Ethylamine is soluble in water whereas aniline is not soluble in water.
(iii) Primary amines have higher boiling points than tertiary amines.(i) Aniline undergoes resonance and as a result, the electrons on the N-atom are delocalized over the benzene ring. Therefore, the electrons on the N-atom are less available to donate.
On the other hand, in case of methylamine (due to the +I effect of methyl group), the electron density on the N-atom is increased. As a result, aniline is less basic than methylamine. Thus, pKb of aniline is more than that of methylamine.
(ii) Ethylamine when added to water forms intermolecular H-bonds with water. Hence, it is soluble in water.
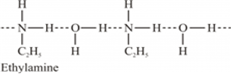
But aniline does not undergo H-bonding with water to a very large extent due to the presence of a large hydrophobic -C6H5 group. Hence, aniline is insoluble in water.
(iii) The boiling points of compounds depend on the extent of H-bonding present in that compound. The more extensive the H-bonding in the compound, the higher is the boiling point. Tertiary amines [(CH3)3N] does not contain H-atom whereas primary amine (CH3NH2) contains two H-atoms. Then, primary amines undergo more extensive H-bonding than tertiary amines. Hence, the boiling point of primary amines is higher than that of tertiary amines.
The conversion of primary aromatic amines into diazonium salts is known as __________ .
Account for the following:
(i) Primary amines (R-NH2) have higher boiling point than tertiary amines (R3N).
(ii) Aniline does not undergo Friedel - Crafts reactions:
(iii) (CH3)2NH is more basic than (CH3)3N in an aqueous solution.
OR
Give reasons :
Although –NH2 is o/p directing group, yet aniline on nitration gives a significant amount of m-nitroaniline.
