Short Answer Type
Short Answer TypeState reasons for the following:
(i) pKb value for aniline is more than that for methylamine.
(ii) Ethylamine is soluble in water whereas aniline is not soluble in water.
(iii) Primary amines have higher boiling points than tertiary amines.The conversion of primary aromatic amines into diazonium salts is known as __________ .
Account for the following:
(i) Primary amines (R-NH2) have higher boiling point than tertiary amines (R3N).
(ii) Aniline does not undergo Friedel - Crafts reactions:
(iii) (CH3)2NH is more basic than (CH3)3N in an aqueous solution.
OR
Give reasons :
Although –NH2 is o/p directing group, yet aniline on nitration gives a significant amount of m-nitroaniline.
Give reasons:
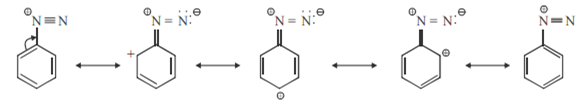
Aromatic diazonium salts are more stable than aliphatic diazonium salts.
Aromatic diazonium salts are more stable than aliphatic diazonium salts. The stability of aromatic diazonium salts is due to resonance which is absent in aliphatic diazonium salts.