 Multiple Choice Questions
Multiple Choice QuestionsIn the chemical reactions,
the compounds 'A' and 'B' respectively are
nitrobenzene and fluorobenzene
phenol and benzene
benzene diazonium chloride and flurobenzene.
benzene diazonium chloride and flurobenzene.
Which of the following compounds will the significant amount of meta product during mono-nitration reaction?


C.
(a) Nitration reactions take place in presence of concentrated HNO3+ concentrated H2SO4.
(b) Aniline acts as a base. In presence of H2SO4, its protonation takes place and anilinium ion is formed
(c)Anilinium ion is a strongly deactivating group and meta directing in nature so it gives meta nitration product in a significant amount.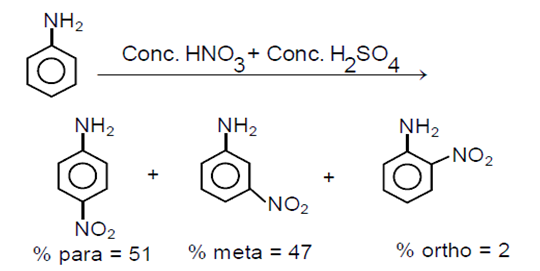
The hydrocarbon which can react with sodium in liquid ammonia is
CH3CH2CH2C≡CCH2CH2CH3
CH3CH2C≡CH
CH3CH=CHCH3
CH3CH=CHCH3
In the chemical reaction, CH3CH2NH2 + CHCl3 + 3KOH → (A) + (B) + 3H2O, the compounds (A) and (B) are respectively
C2H5CN and 3KCl
CH3CH2CONH2 and 3KCl
C2H5NC and K2CO3
C2H5NC and K2CO3
Which one of the following is the strongest base in aqueous solution?
Trimethylamine
Aniline
Dimethylamine
Dimethylamine
Which one of the following methods is neither meant for the synthesis nor for separation of amines?
Hinsberg method
Hofmann method
Wurtz reaction
Wurtz reaction
Amongst the following the most basic compound is
benzylamine
aniline
acetanilide
acetanilide
Which one the following does not have sp2 hybridized carbon?
Acetone
Acetamide
Acetonitrile
Acetonitrile
Consider the acidity of the carboxylic acids
PhCOOH
p – NO2C6H4COOH
o – NO2C6H4COOH
o – NO2C6H4COOH
