 Multiple Choice Questions
Multiple Choice QuestionsAn organic compound X is oxidised by using acidified K2Cr2O7. The product obtained reacts with phenyl hydrazine, but does not answer silver mirror test. The possible structure of X is
CH3COCH3
(CH3)2CHOH
CH3CHO
CH3CH2OH
The increasing order of the rate of HCN addition to compound A-D is
A. HCHO B. CH3COCH3
C.PhCOCH3 D. PhCOPh
A< B < C < D
D< B< C< A
D < C < B < A
C < D< B< A
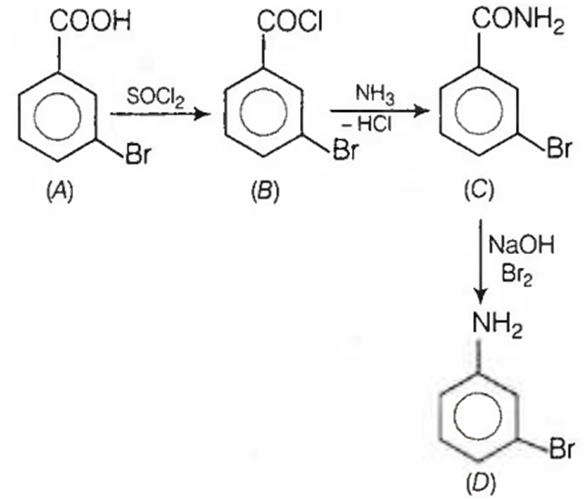
In a set of reactions m - bromobenzoic acid gave a product D . Identify the product D





D.

If 'A' is C2H5NH2, 'B' is (C2H5)NH, 'C' is (C2H5)3N, then order of solubility in water is
A >B > C
B < A <C
C < B <A
C > B <A
The major product (P) in the following reaction is
CH3CH2I (A) (B) (C)
CH3CH2.NH2
CH3CH2.CO.NHBr
CH3.CH2.COONH4
CH3.CH2.CO.NBr2
Amine is not formed in the reaction
(i) hydrolysis of RCN
(ii) reduction of RCH = NOH
(iii) hydrolysis of RNC
(iv) hydrolysis of RCONH2
(i), (ii) and (iv)
(i) and (iv)
(ii) and (iii)
(i), (ii) and (iii)
How many sigma and pi-bonds are there in the molecule of dicyanoethene (CN-CH =CH-CN) ?
3 sigma and 3 pi
5 sigma and 2 pi
7 sigma and 5 pi
2 sigma and 3 pi
Select the correct statement(s).
LiAlH4 reduces methyl cyanide to methyl amine.
Alkaline nitrile has electrophilic as well as nucleophilic centres
Saponification is a reversible reaction.
Alkaline hydrolysis of methane nitrile forms methanoic acids.
