 Multiple Choice Questions
Multiple Choice QuestionsThe correct IUPAC name of [Co(NH3)3(NO2)3] is
Triammine trinitro-N cobalt (III)
Triammine trinitro-N cobalt (II)
Triammine cobalt (III) nitrite
Triammine trinitro-N cobaltate (III)
The replacement of diazonium group by fluorine is known as
Gattermann reaction
Sandmeyer reaction
Balz-Schiemann reaction
Etard reaction
An organic compound X having molecular formula C3H11N reacts with p-toluene sulphonyl chloride to form a compound Y that is soluble in aqueous KOH. Compound X is optically active and reacts with acety lchloride to form compound Z. Identify the compound Z
CH3CH2CH2CH2NHCOCH3
CH3CH2CH(CH3)NHCOCH3
CH3(CH3)CHCH2NHCOCH3
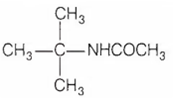
Diethyl amine when treated with nitrous acid yields
diethyl ammonium nitrite
ethyl alcohol
N-nitroso diethyl amine
triethyl ammonium nitrite
The amine 'A' when treated with nitrous acid gives yellow oily substance. The amine A is
triethylamine
trimethylamine
aniline
methylphenylamine
In case of substituted aniline the group which decreases the basic strength is
-OCH3
-CH3
-NH2
-C6H5
Which of the following statement(s) is/are incorrect in case of Hofmann bromamide degradation?
Reaction is useful for decreasing length of carbon chain by one carbon atom
It gives tertiary amine
It gives primary amine
Aqueous or alco. KOH is used with bromine
B.
It gives tertiary amine
Among all the given statements, statement b is incorrect. The given statement is used to synthesise primary amine.
In this reaction, amide on reaction with Br2 in an aqueous or ethanolic solution of NaOH give 1° amine with one C atom less than that present in the amide.
RCONH2 + Br2 + 4KOH RNH2 + 2KBr + K2CO3 + 2H2O
The amine, which reacts with p-toluenesulphonyl chloride to give a clear solution, which on acidification gives insoluble compound is
C2H5NH2
(C2H5)2NH
(C2H5)3N
CH3NHC2H5
Which one of the following can be prepared by Gabriel phthalimide synthesis?
Aniline
o- toluidine
Benzylamine
N-methylethanamine
