 Multiple Choice Questions
Multiple Choice Questions0ne mole of hydrazine (N2H4) loses 10 moles of electrons in a reaction to form a new compound X. Assuming that all the nitrogen atoms in hydrazine appear in the new compound, what is the oxidation state of nitrogen in X? (Note - There is no change in the oxidation state of hydrogen in the reaction).
-1
-3
+3
+5
Which one of the following is used as a test for aliphatic primary amines?
Tollen's test
Fehling's test
lsocyanide test
Azo dye test
When methanamine is treated with benzoyl chloride, the major product is
N-phenylethanamide
N-methylbenzamide
benzanilide
acetophenone
Phenyl isocyanide is prepared from aniline by
Carbylamine reaction
Rosenmund's reaction
Koble's reaction
Reimer-Tiemann reaction
Gabriel's phthalimide synthesis can be used to prepare
ethanamine
N-methylmethanamine
benzene amine
N, N-dimethylmethanamine
Select the compound which on treatment with nitrous acid liberates nitrogen.
Nitroethane
Triethylamine
Diethylamine
Ethylamine
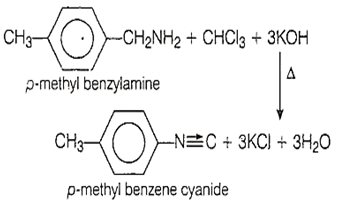
Positive carbylamine test is shown by
N, N-dimethylaniline
triethylamine
N-methylaniline
p-methylbenzylamine
D.
p-methylbenzylamine
Primary amines on heating with chloroform and alcoholic potassium hydroxide solution yield the corresponding isocyanides (carbylamines).
Ethanoic acid on heating with ammonia forms compound A which on treatment with bromine and sodium hydroxide gives compound B. Compound B on treatment with NaNO3/dil.HCl gives compound C. The compounds A, B and C respectively are
ethanamide, methanamine, methanol
propanamide, ethanamine, ethanol
N-ethylpropanamide, methaneisonitrile, methanamine
ethanamine, bromoethane, ethanediazoniumchloride
n-butylamine (l), diethylamine (II) and N, N-dimethylethylamine (III) have the same molar mass. The increasing order of their boiling point is
III < II < I
I < II < III
II < III < I
II < I < III
