 Multiple Choice Questions
Multiple Choice QuestionsChoose the incorrect statement.
Primary amines show intermolecular hydrogen bonds
Tert-butylamine is a primary amine
Tertiary amines do not show intermolecular hydrogen bonds
Isopropylamine is a secondary amine
Amine that cannot be prepared by Gabriel phthalimide synthesis is
aniline
benzylamine
methylamine
iso-butylamine
The correct order of increasing basic nature of the following bases is
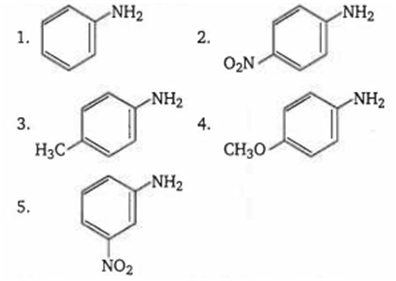
2 < 5 < 1 < 3 < 4
5 < 2 < 1 < 3 < 4
2 < 5 < 1 < 4 < 3
5 < 2 < 1 < 4 < 3
Choose the amide which on reduction with LiAlH4 yields a secondary amine
ethanamide
N-methylethanamide
N, N-dimethylethanamide
phenylmethanamide
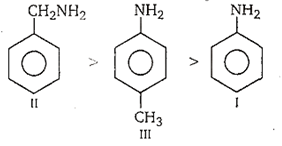
Arrange the following amines in the decreasing order of their basic strength. Aniline (I), Benzylamine (II), p-toluidine (III)
I > II > III
II > III > I
III > II > I
II > I > III
B.
II > III > I
Among the given compounds, benzylamine (C6H5CH2NH2) is the most basic because in it lone pair of nitrogen is not involved in resonance, it is available for donation.
p-toluidine, due to the presence of electron releasing group, (-CH3 group) is more basic than aniline but less basic than benzylamine. Hence, the order of basic strength is
Which one of the following compounds will dissolve in an alkali solution after it has undergone reaction with Hinsberg reagent?
CH3NH2
(CH3)3N
(C2H5)2NH
C6H5NHC6H5
Identify the product in the following sequence
3, 4, 5-tribromobenzene
1, 2, 3-tribromobenzene
2, 4, 6-tribromobenzene
3, 4, 5-tribromonitrobenzene
Among the amines
(A) C6H5NH2
(B) CH3NH2
(C) (CH3)3N
(D) (CH3)2NH
A < B < D <C
D < C < B <A
A > B > C >D
B < C < D <A
