 Multiple Choice Questions
Multiple Choice QuestionsIn the following reaction, X and Y are respectively
CH3COOH + NH3 → X Y + H2O
CH3CONH2, CH4
CH3COONH4, CH3CONH2
CH3CONH2, CH3COOH
CH3NH2, CH3CONH2
Which one of the following is the molecular formula of a tertiary amine ?
C2H7N
C3H9N
CH5N
CH3N
Which one of the following compounds forms a quaternary salt on reacting with excess methyl iodide?
C2H5OCH3
(CH3)2CHOC2H5
C6H5NH2
C6H5NO2
Nitrobenzene on reduction with zinc and NH4Cl gives
azobenzene
aniline
hydrazobenzene
N-phenyl hydroxylamine
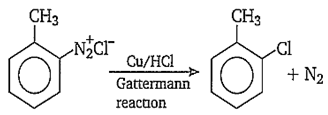
In Gattermann reaction, a diazonium group is replaced by using . and are-
- ; - Cu/ HCl
- ; - CuCl2/ HCl
- ; - CuCl2/ HCl
- Cl2 ; - Cu2O/ HCl
A.
- ; - Cu/ HCl
In Gatterman reaction, are- and Cu/ HCl respectively.
Yellow dye can be prepared by a coupling reaction of benzene diazonium chloride in acidic medium with X. Identify X from the following.
Aniline
Phenol
Cumene
Benzene
Acid hydrolysis of methyl isocyanide gives
CH3NH2 + HCOOH
CH3NH2 + CH3COOH
CH3NH2 + CH3CH2COOH
CH3NH2 + (CH3)2CHCOOH
Mark the correct statement.
Methyl amine is acidic.
Methyl amine is less basic than NH3.
Methyl amine is a stronger base than NH3.
Methyl amine forms salts with bases.
