 Multiple Choice Questions
Multiple Choice QuestionsEthylamine (C2H5NH2) can be obtained from N-ethipthalimide on treatment with
CaH2
H2O
NaBH4
NH2NH2
Aniline dissolved in dilute HCl is reacted with sodium nitrite at 0ºC. This solution was added dropwise to a solution containing equimolar mixture of aniline and phenol in dil. HCl. The structure of the major product is
![]()
![]()
![]()
![]()
Which of the following is NOT a correct method if the preparation of benzylamine from cyanobenzene ?
H2/ Ni
(i) LiAlH4
(ii) H3O+
(i) SnCl2 + HCl(gas)
(ii) NaBH4
(i) HCl/H2O
(ii) NaBH4
The correct statement regarding the basicity of arylamines is
Arylamines are generally more basic than alkylamines because the nitrogen lone pair electrons are not delocalized by interaction with the aromatic ring pi-electron system
Arylamines are generally more basic than alkylamines, because of aryl group
Arylamines are generally more basic than alkylamines because the nitrogen atom in arylamines is sp-hydridized.
Arylamines are generally more basic than alkylamines because the nitrogen atom in arylamines is sp-hydridized.
D.
Arylamines are generally more basic than alkylamines because the nitrogen atom in arylamines is sp-hydridized.
In the aryl amines, due to the delocalization of lone pair of electrons of N-atom to the benzene ring, it loses its basicity and becomes less basic than alkyl amine.
on the other hand, alkyl amines have +I alkyl effect of the alkyl group which increase electron density on N -atom. Hence the availability of free electron on amine as well as +I effects enhances its basic nature.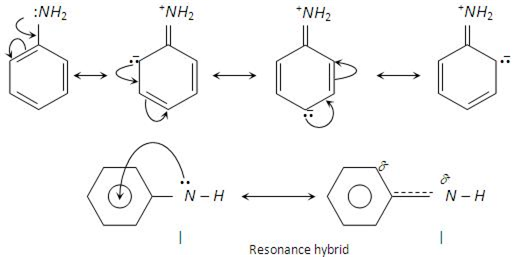
In the reaction,
X and Y are
X=2-butyne; Y= 3-hexyne
X=2-butyne;Y=2-hexyne
X=1-butyne ;Y = 2-hexyne
X=1-butyne ;Y = 2-hexyne
Consider the nitration of benzene using mixed conc. H2SO4 and HNO3. If a large amount of KHSO4 is added to the mixture, the rate of nitration will be
slower
uncharge
doubled
doubled
The product formed by the reaction of an aldehyde with a primary amine is
Ketone
Carboxylic acid
Aromatic acid
Aromatic acid
The electrolytic reduction of nitrobenzene in strongly acidic medium produces
p-aminophenol
azoxybenzene
azobenzene
aniline
