 Multiple Choice Questions
Multiple Choice QuestionsAniline when diazotised in cold and then treated with dimethyl aniline gives a coloured product. The structure of coloured product would be:
![]()
![]()
![]()
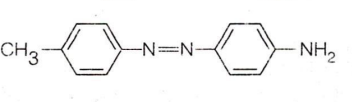
In the given reaction,
 X Y
X Y
The products X and Y respectively are:
o-bromo nitrobenzene and o-bromoaniline
p-bromo nitrobenzene and p-bromoaniline
m-bromo nitrobenzene and m-bromoaniline
m-bromo nitrobenzene and m-bromoaniline
Arrange the following in the increasing order of their basic strengths :
CH3NH2 , (CH3)2NH , (CH3)3N , NH3
NH3 < (CH3)3N < (CH3)2NH < CH3NH2
NH3 < (CH3)3N < CH3NH2 < (CH3)2NH
(CH3)3N < NH3 < CH3NH2 < (CH3)2NH
CH3NH2 < (CH3)2NH < (CH3)3N < NH3
B.
NH3 < (CH3)3N < CH3NH2 < (CH3)2NH
Aliphatic amines are more basic than NH3 due to + I effect of alkyl groups. In aqueous medium, tertiary amine is less basic than secondary amine, because the cation formed by protonation of tertiary amine is less solvated as compared to the cation formed by protonation of secondary amine. Hence, the increasing order of the basic strength is as ,
NH3 < (CH3)3N < CH3NH2 < (CH3)2NH
Assertion : Benzene reacts with iodine monochloride in presence of anhyd. AlCl3 to form iodobenzene.
Reason : Iodine monochloride reacts with anhyd. AlCl3 to produce I+ which attacks the benzene ring.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Which amine amongst the following will answer positively the carbylamine test?
C6H5 - NH - CH3
![]()
C6H5 - NH - C4H9
C6H5 - N(C2H5)2
