Short Answer Type
Short Answer TypeWhich vitamin is called ascorbic acid? What are its physiological functions? Which disease is caused by the deficiency of this vitamin?
Explain the following:
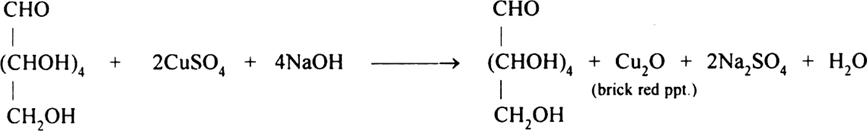
Glucose responds Fehling solution and osazone, it does not react with Schiff’s base and sodium bisulphite.
Explain the following:
Glucosides react neither with Fehling solution nor with Tollen's reagent. They also do not allow mutarotation.
Explain the following:
Although fructose does not have an aldehydic group, it reacts with Fehling solution.
What is melting temperature (Tm) of DNA? A DNA molecule with more number of GC base pairs than AT base pairs has higher Tm than the one with lesser number of GC base pairs than AT base pairs. Explain why?
What are forces holds the two strands of DNA together in a double-helix structure? Draw structure to show the bonding between adenine and thymine, and between guanine and cytosine.
 Long Answer Type
Long Answer Type