 Short Answer Type
Short Answer TypeDefine the following terms as related to proteins:
(i) Peptide linkage
(ii) Primary structure
(iii) Denaturation
After watching a programme on TV about the presence of carcinogens(cancer causing
agents) Potassium bromate and Potassium iodate in bread and other bakery products, Ritu a class XII student decided to aware others about the adverse effects of these carcinogens in foods. She consulted the school principal and requested him to instruct canteen contractor to stop selling sandwiches, pizza, burgers and other bakery products to the students. Principal took an immediate action and instructed the canteen contractor to replace the bakery products with some proteins and vitamins rich food like fruits, salads, sprouts etc. The decision was welcomed by the parents and students.
After reading the above passage, answer the following questions :
(i) What are the values (at least two) displayed by Ritu?
(ii) Which polysaccharide component of carbohydrates is commonly present in bread?
(iii) Write the two types of secondary structure of proteins.
(iv) Give two examples of water soluble vitamins.
Amino acids show amphoteric behaviour. Why?
In aqueous solution, the carboxyl group of an amino acid can lose a proton and the amino group can accept a proton to give a dipolar ion known as zwitter ion.
Therefore, in zwitter ionic form, the amino acid can act both as an acid and as a base. Thus, amino acids show amphoteric behaviour.
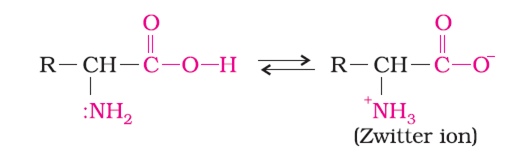
 Multiple Choice Questions
Multiple Choice QuestionsOne mole of stachyose on hydrolysis yields
1 mole of glucose+ 1 mole of fructose+ 2 mole of galactose
2 mole of glucose + 1 mole of fructose + 1 mole of galactose
1 mole of glucose+ 2 mole of fructose+ 1 mole of galactose
2 mole of glucose+ 2 mole of fructose
