 Long Answer Type
Long Answer TypeDiscuss the formation of compounds in which octet rule is violated.
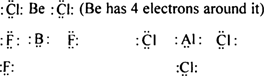

Give the comparison between Electrovalent (or ionic compounds) compounds and covalent compounds (special reference to properties).
Discuss the orbtial concept or quantum concept for the formation of covalent bond.
Or
Explain the formation of covalent bond on the basis of valence bond theory.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeDiscuss the orbital shapes of the following covalent molecules:
(i) H2 (ii) F2 (iii) O2 (iv) N2.
