 Long Answer Type
Long Answer TypeDiscuss the orbital shapes of the following covalent molecules:
(i) HF (ii) H2O (iii) NH3.
 Short Answer Type
Short Answer TypeWhy is a sigma bond stronger than pi bond ?
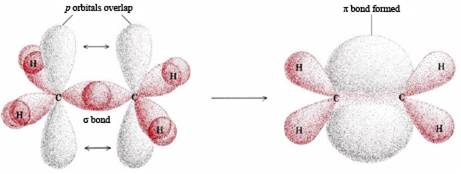
 Long Answer Type
Long Answer TypeUsing the orbital overlap concept, explain the formation of:
(i) O2 molecule (ii) N2 molecule.
What is coordinate or dative bond? Explain the formation of the bond with some examples.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type