 Long Answer Type
Long Answer TypeExplain coordinate bond by the orbital concept.
A coordinate bond is formed by the overlapping of an orbital of an atom containing a lone pair of electrons with the empty orbital of the other atom.
Let us consider the formation of ammonium ion (N+H4). It is formed by the combination of ammonia molecule and hydrogen ion.![]()
The central nitrogen atom of ammonia molecule has one orbital containing a lone pair of electrons while the other three orbitals are filled up with bond pairs of electrons.
On the other hand, hydrogen ion has empty is orbital. When the H+ ion approaches ammonia molecule, its empty orbital overlaps the orbital of nitrogen atom containing a lone pair to form a dative bond. 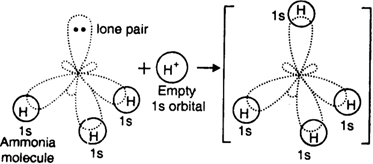
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeName the theory responsible for the definite geometry of covalent molecules. Give main features of the theory ?
 Short Answer Type
Short Answer TypeHow will you explain the following order of the repulsive interactions between pair of electrons on the central atom:
Lone pair - Lone pair > Lone pair - Bond pair > Bond pair - Bond pair.
 Long Answer Type
Long Answer TypeOn the basis of VSEPR theory, discuss the geometry of the following covalent molecules: (i) BeF2 (ii) BF3 (iii) CH4.
 Short Answer Type
Short Answer TypeAlthough geometries of NH3 and H2O molecules are distorted tetrahedral, the bond angle in water is less than that of ammonia. Discuss.
