 Long Answer Type
Long Answer TypeDiscuss the shape of the following molecules using VSEPR model:
BeCl2, BCl3, SiCl4, AsF5, H2S, PH3
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeDiscuss in brief sp2 hybridization (hybridization in C = C bond). Discuss the molecular orbital structure of ethylene (first member of alkene).
Or
Draw diagrams showing the formation of a double bond between carbon atoms in C2H4.
 Short Answer Type
Short Answer TypeApart from tetrahedral geometry, another possible geometry for CH4 is square planar with four H atoms at the corners of the square and the C atom at its centre. Explain why CH4 is not square planar?
 Long Answer Type
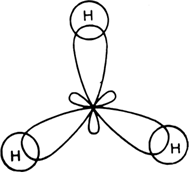
Long Answer TypeOn the basis of hybridisation, discuss the orbital structure of: (i) BeF2 (ii) BH3.
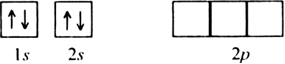
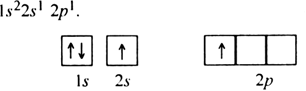


 Short Answer Type
Short Answer TypeThe central atoms in CH4, NH3 and H2O are all said to have similar hybridisation but the bond angle H – A – H (where A is C, N or O) is different in each case. Explain stating in which case it is maximum and in which case it is minimum.
