 Long Answer Type
Long Answer TypeState and explain the geometric arrangements possible in sp3d and sp3d2 hybridisation. Name the d-orbitals involved in these.
Which hybrid orbitals are used by carbon atoms in the following molecules ?
(a) CH3 – CH3
(b) CH3 – CH = CH2
(c) CH3 – CH2 - OH
(d) CH3 - CHO
(e) CH3COOH
 Short Answer Type
Short Answer TypeDescribe the change in hybridization (if any) of the Al atom in the following reaction:![]()
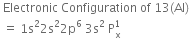
Is there any change in the hybridisation of B and N atoms as a result of the following reaction?
BF3 + NH3 → F3B.NH3?
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type