 Short Answer Type
Short Answer TypeWhat causes the two atoms to combine to form a bond which is:
(i) Non-polar
(ii) Polar-covalent and
(iii) Ionic in nature?
 Long Answer Type
Long Answer TypeBriefly explain the term 'Dipole moment'. How is it expressed and what are its units?
Although both CO2 and H2O are triatomic molecules, the shape of H2O molecule is bent While that of CO2 is linear. Explain this on the basis of dipole moment.
The CO2 molecule has a zero dipole moment even though C and O have different electronegativities and each of the C = O bond is polar and has the same dipole moment. This indicates that the individual dipole moments are equal in magnitude and pointed in opposite directions and as a result, they cancel out each other.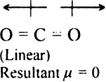
On the other hand, water is a polar molecule having a dipole moment, 1.84D. It is because water has been a bent structure in which two O-H bonds are oriented at an angle of 104.5° and do not cancel the dipole moments of each other. The molecular dipole moment of water (μ =1.84 D) is the resultant of the individual values of the dipole moment of two O – H bonds.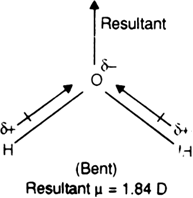
 Short Answer Type
Short Answer TypeExplain why BeH2 molecule has a zero dipole moment although the Be-H bonds are polar?
 Long Answer Type
Long Answer TypeSketch the bond moments and resultant dipole moment in SO2, cis and trans forms of CH2Cl2.
 Short Answer Type
Short Answer Type