 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeThe molecule of SO2 has a dipole moment. Is the molecule linear or bent? Explain your answer.
Predict the dipole moment of;
(i) a molecule of the type AX4 having a square planar geometry.
(ii) a molecule of the type AX5 having a trigonal bipyramdial.
(iii) a molecule of the type AX6 having an octahedral geometry.
 Long Answer Type
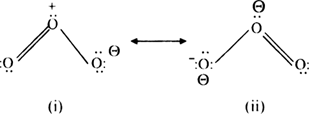
Long Answer TypeDiscuss the resonance in CO2 and O3molecule.
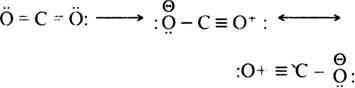
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type