 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeThe molecule of SO2 has a dipole moment. Is the molecule linear or bent? Explain your answer.
Predict the dipole moment of;
(i) a molecule of the type AX4 having a square planar geometry.
(ii) a molecule of the type AX5 having a trigonal bipyramdial.
(iii) a molecule of the type AX6 having an octahedral geometry.
 Long Answer Type
Long Answer TypeWrite the resonance structures for SO3, NO2 and ![]()
Resonance structure of the given molecule,
(i) SO3 :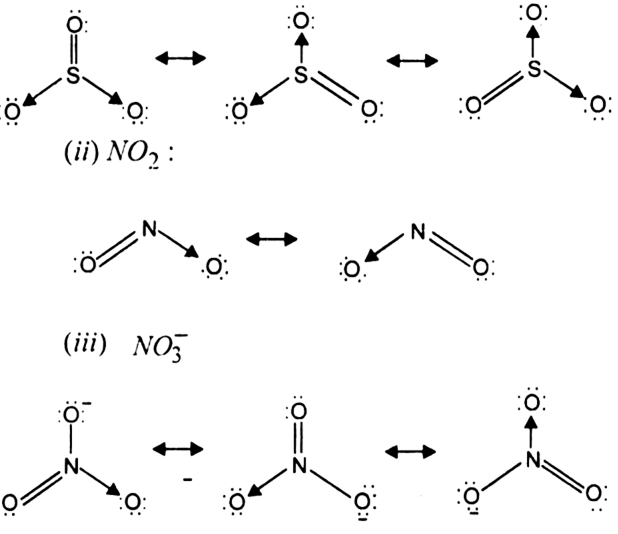
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type