 Long Answer Type
Long Answer TypeDiscuss the formation of N2 molecule on the basis of MO theory. Predict its:
(i) Bond order
(ii) Magnetic character.
Write the stability configuration of ![]() and predict their bond order, stability and magnetic character.
and predict their bond order, stability and magnetic character.
Draw the molecular orbital energy diagram for oxygen molecule (O2) and show that:
(i) It has a double bond
(ii) It has paramagnetic character.
Using a molecular orbital diagram, predict the bond order, stability and magnetic character of O2–,O2+, and  . Also, write their electronic configurations.
. Also, write their electronic configurations.
Discuss the relatives stabilities, bond dissociation energies and bond lengths of O2,  species.
species.
For O2 molecule:
Nb = 8, Na = 4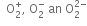
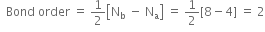
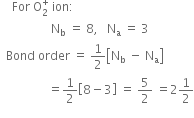
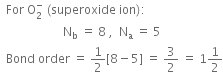


As bond dissociation energies are directly proportional to the bond order, therefore, the dissociation energies of these molecular species are in order: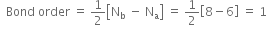
As greater the bond dissociation energy, greater is the stability; the stability of these species is also as in the above order.
As bond length is inversely proportional to bond order, therefore their bond lengths will be in order: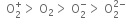
Is the bond order in superoxide ion more or less than in peroxide ion? Explain on the basis of MO theory.
 Short Answer Type
Short Answer TypeUsing bond order show that N2 would be expected to have a triple bond, F2 a single bond and Ne2 no bond.
Show that N2 molecule has a greater bond dissociation energy than N-2 where O2 has lower bond dissociation energy than O+2.
