 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following is not correct regarding the properties of ionic compounds?
Ionic compounds have high melting and boiling points
Their reaction velocity in aqueous medium is very high
Ionic compounds in their molten and aqueous solutions do not conduct electricity
They are highly soluble in polar solvents
Match the following lists:
| List - I | List - II |
| A. Ethane | 1. sp carbons |
| B. Ethylene | 2. 6 sp2 carbons |
| C. Acetylene | 3. 2 sp3 carbons |
| D. Benzene |
4. 2 sp2 carbons 5. 1 sp and 1 sp2 carbons |
A - 3; B - 4; C - 1; D - 2
A - 4; B - 5; C - 3; D - 2
A - 3; B - 1; C - 2; D - 5
A - 2; B - 3; C - 4; D - 5
Which of the following pair of ions have same paramagnetic moment?
Cu2+, Ti3+
Mn2+, Cu2+
Ti4+, Cu2+
Ti3+, Ni2+
Average C-H bond energy is 416 kJ mol-1. Which of the following is correct?
CH4 (g) + 416 kJ → C (g) + 4H (g)
CH4 (g) → C (g) + 4H (g) + 416 kJ
CH4 (g) + 1664 kJ → C (g) + 4H (g)
CH4 (g) → C (g) + 4H (g) + 1664 kJ
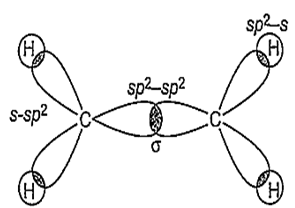
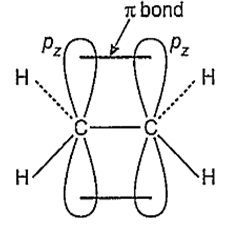
A molecule (X) has
(i) four sigma (σ) bonds formed by the overlap of sp2 and s orbitals
(ii) one sigma (σ) bond formed by sp2 and sp2 orbitals and
(iii) one bond formed by px and pz orbitals.
Which of the following is X?
C2H6
C2H3Cl
C2H2Cl2
C2H4
D.
C2H4
X is C2H4 and its structure is-
i. Formation of σ bond:
ii. Formation of bond:
iii. Geometry of the molecule:
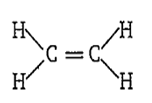
The bond length of HCl molecule is 1.275 Å and its dipole moment is 1.03 D. The ionic character of the molecule (in percent) (charge of the electron = 4.8 × 10-10 esu) is
100
67.3
33.66
16.83
Which one of the following is a correct set ?
H2O, sp3, angular
BCl3, sp3, angular
NH, dsp2, square planar
CH4, dsp2, tetrahedral
