 Multiple Choice Questions
Multiple Choice QuestionsMatch the following
| Column I (molecules) | Column II ( number of lone pair on central atom) | ||
| A | NH3 | 1 | Three |
| B | H2O | 2 | Two |
| C | XeF2 | 3 | Zero |
| D | CH4 | 4 | Four |
| 5 | One |
A B C D
5 1 3 2
A B C D
3 1 2 5
A B C D
5 1 2 3
A B C D
1 5 3 4
Identify the order in which the spin only magnetic moment (in BM) increases for the following four ions
(I) Fe2+
(II) Ti2+
(III) Cu2+
(IV) V2+
I, II, IV, III
IV, I, II, III
III, IV, I, II
III, II, IV, I
The formal charges of N(1), N(2) and O atoms in the following figure are respectively
![]()
+1, -1, 0
-1, +1, 0
+1, +1, 0
-1, -1, 0
In which of the following pairs, the central atoms have the same number of lone pairs of electrons?
PCl5, BrF5
XeF2, ICl
XeF4, ClO
SCl4, CH4
B.
XeF2, ICl
Both XeF2 and ICl molecule have three lone pairs of electron on central atom.

Hence, in XeF2 three lone pairs are present.
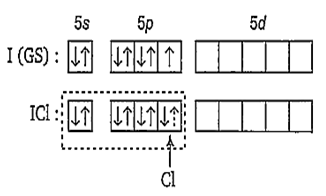
Hence, ICl three lone pairs are present.
Identify the correct set.
| Molecule | Hybridisation of central atom | Shape |
| PCl5 | dsp3 | square pyramidal |
| [Ni(CN)4]2- | sp3 | tetrahedral |
| SF6 | sp3d2 | octahedral |
| IF3 | dsp3 | pyramidal |
Which one of the following statements is correct?
Hybrid orbitals do not form σ bonds.
Lateral overlap of p-orbitals or p- and d-orbitals produces -bonds.
The strength of bonds follows the order-
σp-p < σs-s < p-p
s-orbitals do not form σ bonds.
According to molecular orbital theory, the total number of bonding electron pairs in O2 is
2
3
5
4
In which one of the following pairs the two species have identical shape, but differ in hybridisation?
I, BeCl2
NH3, BF3
XeF2, I
NH, SF4
