 Multiple Choice Questions
Multiple Choice QuestionsThe bond present in N2O5 are
only ionic
covalent and coordinate
only covalent
covalent and ionic
B.
covalent and coordinate
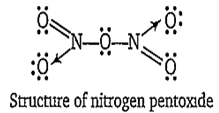
Nitrogen pentoxide N2O5) has both covalent as well as coordinate bonds. In the gaseous state, it exists as a symmetrical molecule having the structure O2N-O-NO2. The N-O-N bond is almost linear. X-ray studies reveals the ionic nature ofsolid N2O5 i.e., nitronium nitrate, NaNO.
Which of the following statements is false?
1. Non-bonding pairs occupy more space than bonding pairs.
2. The bonding orbitals in trigonal bipyramidal molecule are described as sp3d hybrids.
3. SnCl2 has linear shape.
4. PCl and AlCl are isoelectronic.
1
2
3
4
The correct order of increasing C-O bond length of CO, CO2 and CO is
CO < CO2 < CO
CO2 < CO < CO
CO < CO < CO2
CO < CO2 < CO
Covalent compounds have low melting point because
covalent molecules are held by weak van der Waal's force of attraction
covalent bond is less exothermic
covalent bond is weaker than ionic bond
covalent molecules have definite shape
Chemical bond implies
attraction and repulsion
attraetion and repulsion balanced at a particular distance
attraction
repulsion
The percentage s-character of the hybrid orbitals in methane, ethene and ethyne are respectively
25, 33, 50
25, 50, 75
50, 75, 100
10, 20, 40
For a stable molecule, the value of bond order must be
there is no relationship between stability and bond order
zero
positive
negative
