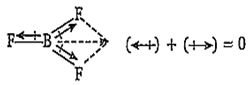Multiple Choice Questions
Multiple Choice QuestionsAll bond angles are exactly equal to 109° 28' in:
methyl chloride
iodoform
chloroform
carbon tetrachloride
The molecular shapes of SF4, CF4 and XeF4 are
different with 1, 0 and 2 lone pairs of electrons on the central atom, respectively
different with 0, 1 and 2 lone pairs of electrons on the central atom, respectively
the same with 1, 1 and 1 lone pair of electrons on the central atoms, respectively
the same with 2, 0 and 1 lone pairs of electrons on the central atom, respectively
Which one of the following does not have sp2 hybridised carbon ?
Acetone
Acetic acid
Acetonitrile
Acetamide
Among the following, the pair in which the two species are not isostructural, is
SiF4 and SF4
IO and XeO3
BH
PF and SF6
The helical structure of protein is stabilized by
dipeptide bond
hydrogen bonds
ether bonds
peptide bonds
The dipole moment of HBr is 1.6 × 10-30 cm and inter-atomic spacing is 1 Å. The % ionic character of HBr is
7
10
15
27
The molecule having zero dipole moment is
CH2Cl2
BF3
NF3
ClF3
B.
BF3
The dipole moment of BF3 molecule is zero due to its symmetrical (triangular planar) structure. The three fluorine atoms lie at the corners of an equilateral triangle with boron at the centre. Thus, the vectorial addition of the dipole moments of the three bonds gives a netsum of zero.