 Multiple Choice Questions
Multiple Choice QuestionsThe polypeptides are obtained by assembling the peptide units by
ionic bond
covalent bond
intermolecular H-bonding
covalent and H-bonding
Which one of the following have linear structure?
(I) I
(II) NO
(III) I
(IV) SO2
(V) N
I, II and III
I and V
II, III and IV
All of these
B.
I and V
I ion is made up of l2 molecule with an I- bonded to it by means of a coordinate bond. There are two bond pairs and three lone pairs. In the outer shell of central atom. To minimise the repulsive forces the three lone pairs occupy the equatorial positions. The ion is therefore, linear in shape with a bond angle of exactly 180°.
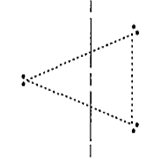
Similarly, N ion is also linear in shape.
The dipole moment of HBr is 1.6 × 10-30 cm and inter atomic spacing is 1 Å. The % ionic character of HBr is
7
10
25
27
The bond order of H If it has 2 bonding electron, how many antibonding electrons it will have?
4
3
2
1
A magnetic moment of 1.73 BM will be shown by which one among the following compounds
[Cu(NH3)4]2+
[Ni(CN)4]2-
TiCl4
[CoCl6]4-
| Iodide | PI3 | AsI3 | SbI3 |
| Bond angle | 102 | 100°2' | 99° |
due to small size of P
due to more bp-bp repulsion in PI3
due to less electronegativity of P
None of the above
The shape of [PtCl3(C2H4)]- and the hybridisation of Pt respectively are
tetrahedral, sp3
trigonal pyramidal, sp3
square planar, dsp2
square planar, d2sp3
Among the following compounds both coloured and paramagnetic one is
K2Cr2O7
VOSO4
(NH4)2.[TiCl2]
K3[Cu(CN)4]
