 Multiple Choice Questions
Multiple Choice QuestionsThe bond length of HCl bond is 2.29 x 10-10 m. The percentage ionic character of HCl, if measured dipole moment is 6.226 x 10-30 C- m, is
8%
20%
17%
50%
What is the wavelength (in m) of a particle of mass 6.62 x 10-29 g moving with a velocity of 103 ms-1?
6.62 x 10-4
6.62 x 10-3
10-5
105
H-O-H bond angle in H2O is 104.5° and not 109° 28' because of
lone pair-lone pair repulsion
lone pair-bond pair repulsion
bond pair-bond pair repulsion
high electronegativity of oxygen
The atomic number of an element 'M' is 26. How many electrons are present in the M-shell of the element in its M3+ state ?
11
15
14
13
In which of the following pairs, both molecules possess dipole moment ?
CO2, SO2
BCl3, PCl3
H2O, SO2
CO2, CS2
C.
H2O, SO2
Among all the given options, H2O and SO2 both possess dipole moment due to bent structure. Their structure is given below:
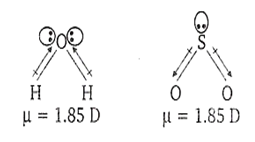
When dihydroxy acetone reacts with HIO4, the product is/ are
HCHO
HCOOH
HCHO and HCOOH
HCHO and CO2
