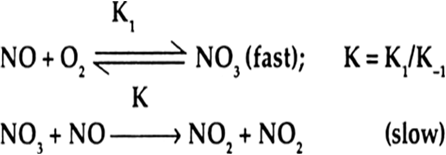121.
For the reaction at 500 K
The proposed mechanism is as follow:
(i)
Predict the law?
Step I:
Step II:
Step II is much faster than step I, that is k2 >> k1 Step I is rate determining step and thus the rate of overall reaction equals to rate of step I. Step I is is a bimolecular process that has the rate law :
126 Views
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type