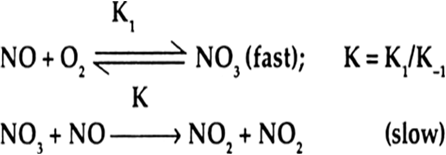Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeThe rate expression is derived by step II of the mechanism, as it is the slower one
rate = k[NOBr2][NO] ...(i)
However, NOBr2 is an intermediate and thus its concentration should be replaced from equation (i)
From step (i),
Equilibrium constant,
...(ii)
Then by equation (i) and (ii)
Order of the reaction is 2+1 =3
 Long Answer Type
Long Answer Type