 Multiple Choice Questions
Multiple Choice QuestionsIn a zero order reaction for every 10o rise of temperature, the rate is doubled. If the temperature is increased from 10oC to 100oC, the rate of the reaction will become
256 times
512
64 times
128 times
Activation energy (Ea) and rate constants (k1 and k2) of chemical reaction at two different temperatures (T1 and T2) are related by




Which one of the following statements for the order of a reaction is correct?
Order is not influenced by stoichiometric coefficient of the reactants
Order of reaction is sum of power to the concentration terms of reactants to express the rate of reaction
Order of reaction is always the whole number
Order of reaction is always the whole number
The rate of constant of the reaction A → B is 0.6 x 10-3 mole per second. If the concentration of A is 5 M then concentration of B after 20 min is
1.08 M
3.60 M
0.36 M
0.36 M
The half -life of a substance in a certain enzyme catalysed reaction is 138 s. The time required for the concentration of the substance to fall from 1.28 mg L-1 is
414 s
552 s
690 s
690 s
The rate of reaction 2N2O3 → 4 NO2 + O2 can be written in three ways
The relationship between k and k' and between k and k'' are
k' = 2k ; k' = k
k' = 2k; k" = k/2
k' = 2k; k' = 2k
k' = 2k; k' = 2k
The unit of rate constant for a zero order reaction is
mol L-1 s-1
L mol-1 s-1
L2 mol-2 s-1
L2 mol-2 s-1
For the reaction
the value of the rate of disappearance of N2O5 is given as
6.25 x 10-3 mol L-1s-1 and 6.25 x 10-3 mol L-1 s-1
1.25 x 10-2 mol L-1s-1 and 6.25 x 10-3 mol L-1 s-1
6.25 x 10-3 mol L-1s-1 and 3.125 x 10-3 mol L-1 s-1
6.25 x 10-3 mol L-1s-1 and 3.125 x 10-3 mol L-1 s-1
For an endothermic reaction, energy of activation is Ea and enthalpy of reaction is ΔH (both of these in kJ/mol). Minimum value of Ea will be
less than ΔH
equal to ΔH
more than ΔH
more than ΔH
C.
more than ΔH
In endothermic reactions, the energy of reactants is less than that of the products. Potential energy diagram for endothermic reactions is,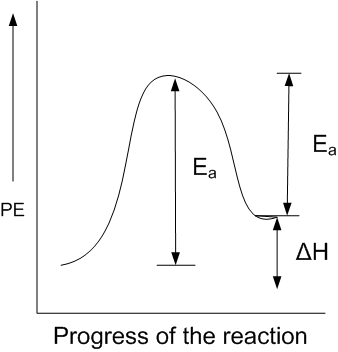
Where Ea = activation energy of forwarding reaction
Ea' = activation energy of backwards reaction
ΔH = enthalpy of the reaction
From the above diagram,
Ea = Ea' + ΔH
Thus, Ea > ΔH
During the kinetic study of the reaction, 2A + B --> C+ D, following results were obtained
|
|
[A]/mol L- |
[B]/ mol L- |
Initial rate of formation of D/ mol L- min- |
|
I |
0.1 |
0.1 |
6.0 x 10-3 |
|
II |
0.3 |
0.2 |
7.2 x 10-2 |
|
III |
0.3 |
0.4 |
2.88 x 10-1 |
|
IV |
0.4 |
0.1 |
2.40 x10-2 |
rate = k[A]2[B]
rate = k [A][B]
rate = k[A]2[B]2
rate = k[A]2[B]2
