 Short Answer Type
Short Answer TypeThe first ionisation enthalpies in electron volts of nitrogen and oxygen atoms are respectively given by:
(i) 14.6, 13.6
(ii) 13.6, 14.6
(iii) 13.6, 13.6
(iv) 14.6, 14.6
Al atom loses electrons successively to form Al+, Al2+ and Al3+ ions. Which step will have highest ionsiation enthalpy?
The size of isoelectronic species: F-, Ne and Na+ is affected by:
(a) nuclear charge (Z)
(b) valence principal quantum number (n)
(c) electron-electron interaction in the outer orbitals.
(d) none of the factors because their size in the same.
From the electronic configuration of the elements A, B and C given below: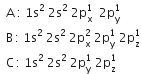
(i) Which one has least negative electron gain enthalpy and
(ii) Which one has highest ionisation enthalpy value?
