 Short Answer Type
Short Answer TypeWhich elements occupy the maxima of ionisation potential curve?
Noble gases elements occupy the maxima of ionisation potential curve.
The first ionisation enthalpies in electron volts of nitrogen and oxygen atoms are respectively given by:
(i) 14.6, 13.6
(ii) 13.6, 14.6
(iii) 13.6, 13.6
(iv) 14.6, 14.6
Al atom loses electrons successively to form Al+, Al2+ and Al3+ ions. Which step will have highest ionsiation enthalpy?
The size of isoelectronic species: F-, Ne and Na+ is affected by:
(a) nuclear charge (Z)
(b) valence principal quantum number (n)
(c) electron-electron interaction in the outer orbitals.
(d) none of the factors because their size in the same.
From the electronic configuration of the elements A, B and C given below: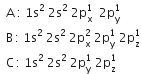
(i) Which one has least negative electron gain enthalpy and
(ii) Which one has highest ionisation enthalpy value?
