 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeDiscuss the earlier attempts to classify elements.
Different scientists tried to group elements with similar elements.
(i) Dobereiner Triads (1822). Dobereiner arranged similar elements in a group of three so that the atomic mass of the central element was nearly equal to arithmetic mean of the atomic mass of the other two elements. The group of three elements was named as triad. For example,
Triad Lithium Sodium Potassium Mean atomic mass
Atomic masses 7 23 39 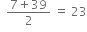
Triad Chlorine Bromine Iodine Mean atomic mass
Atomic masses 35.5 80 127 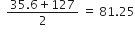
But this concept of triad could be applied to a limited number of elements. It was rejected soon.
(ii) Newland's law of octaves (1864). Newland observed that if the known elements were arranged in order of their increasing atomic masses, similar properties recurred in every eighth element like nodes in a musical scale. This generalisation was known as Law of octaves. This system worked very well for lighter elements.![]()
But this law failed in case of heavier elements and was rejected.
(iii) Lother Meyer’s work (1870). Lother Meyer plotted a graph between atomic volumes of elements in the solid state and their atomic masses. He found that the elements with similar physical properties occupied similar positions on the curve. By taking into account this fact, he drew up a periodic table in which elements were arranged in order of increasing atomic masses.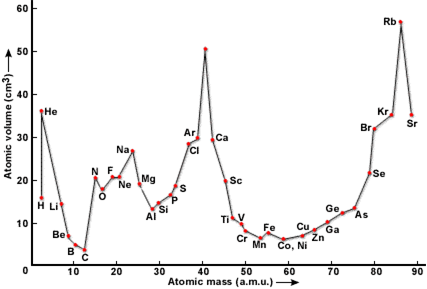
 Short Answer Type
Short Answer TypeWhich important property did Mendeleev use to classify the elements in his Periodic Table? and did he stick to that?
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type