 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeAccount for the fact that the 4th period has eighteen and not eight elements.
In the long form of the periodic table, each period starts with the filling up of a new principal shell. Clearly, the 4th period will begin with filling of 4th shell i.e. N shell. Thus, fourth period starts with the filling up of 4s orbtial (n = 4). After filling 4s orbital, the filling of 3rd and then 4p takes place. It is so because enthalpy of 3d sub level in between 4s and 4p sub levels. As,
4s = 2 electrons
3d = 10 electrons 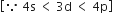
4p = 6 electrons
_______________
18 electrons
Therefore, 18 elements (not eight) are present in the 4th period. The 4d and 4f sublevels are higher in enthalpy than 5s and hence are filled up in the next periods.
 Short Answer Type
Short Answer TypeLanthanoides and actinodes are placed in separate rows at the bottom of the periodic table. Explain the reason for this arrangement.
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeName the block to which the elements with following valence shell electronic configurations belong:
(i) 3s2 3p5
(ii) 3d104s2
(iii) 3p64s2
(iv) 6s2 4f0
(v) 4s1 3d5
