 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWhat are successive ionisation enthalpies? Explain why the second ionisation enthalpy is higher than the first ionisation enthalpy?
 Short Answer Type
Short Answer TypeWhat are the various factors due to which the ionization enthalpy of the main group elements tends to decrease down a group?
Why does the first ionsiation enthalpy increase as we go from left to right through a period of the periodic table?
Or
How does ionisation enthalpy vary along a period?
Why ionsiation enthalpy of N is more than that of O even though oxygen has higher nuclear charge than nitrogen?
This is due to the fact that electronic configuration of nitrogen in which all 2p orbitals,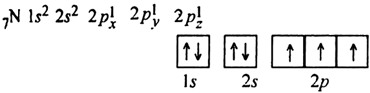
are half filled, is much more stable than that of oxygen,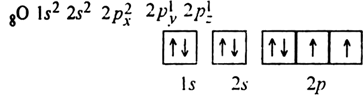
in which all the 2p orbitals are neither half filled nor fully filled. Therefore. IE1 of N is more than that of oxygen.
