10.
Explain on the basis of valence bond theory that [Ni(CN)4]2– ion with square planar is diamagnetic and the [NiCl4]2– ion with tetrahedral geometry is paramagnetic.
(i) [Ni(CN)4]2–
Ni is in the +2 oxidation state i.e., in d
8 configuration.
There are 4 CN− ions. Thus, it can either have a tetrahedral geometry or square planar geometry. Since CN− ion is a strong field ligand, it causes the pairing of unpaired 3d electrons.
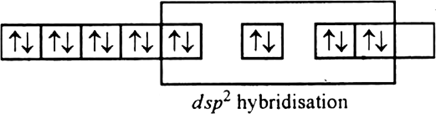 CN– will cause pairing of electrons. It is diamagnetic in nature due to the unpaired electron.
CN– will cause pairing of electrons. It is diamagnetic in nature due to the unpaired electron.
(ii) [Ni(Cl4)]2–
In case of [NiCl4]
2−, Cl
− ion is a weak field ligand. Therefore, it does not lead to the pairing of unpaired 3d electrons. Therefore, it undergoes sp3 hybridization. Since there are 2 unpaired electrons in this case, it is paramagnetic in nature.

2301 Views
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type

