 Short Answer Type
Short Answer TypeWrite the chemical formula of:
(i) Sodium tetrahydridoborate(III).
(ii) Tetrahydroxo zincate(II) ion.
Specify which out of the following complex structures exhibit geometrical isomerism:
(a) Linear, (b) Square planar, (c) Tetrahedral, (d) Octahedral.
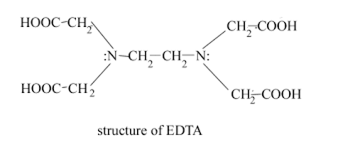
Give an example of hexadentate ligand.
Why is geometrical isomerism not possible in tetrahedral complexes having two different types of unidentate ligands coordinated with the central metal ion ?
