 Short Answer Type
Short Answer TypeGive one example of chelate complex.
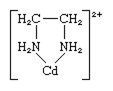
Write the name of any complex of copper in which the oxidation state of copper is one.
Give one example of each complex in which sulphate ion acts as mondenate and bidentate ligand.
What is the rule for writing the names of different ligands (neutral, negative etc.) during the naming of complexes?
 Multiple Choice Questions
Multiple Choice Questions Short Answer Type
Short Answer TypeWhat will be the correct for the wavelengths of absorption in the visible region for the following: , [Ni(NO2)6]4- , [Ni(NH3)6]2+, [Ni(H2O)6]2+?
